Solving the Mercury Battery Dilemma
A handy guide to non-toxic workarounds for vintage cameras & meters
By Jason Schneider
Beginning in the early 1960s, battery powered CdS, and later, SBC cells, were steadily replacing bulkier, less sensitive selenium cells in the metering systems built into cameras and handheld exposure meters. Compared to their selenium cell-based counterparts, these new battery powered meters and metering systems worked at far lower light levels, had narrower acceptance angles, making it easier to get accurate readings from shooting position, didn’t require a delicate micro-ammeter, and could be integrated more compactly into camera bodies without the telltale honeycomb grid. However, unlike selenium cells that generate a measurable current on their own when exposed to light these new meters required batteries.
Initially, most camera and meter manufacturers turned to mercury batteries, technically known as mercuric-oxide cells, to power these new meters and metering systems because they offer a host of operational advantages. Mercury batteries deliver a very constant voltage over their long lifespan and then die suddenly, virtually eliminating the potential for inaccurate readings; they don’t require complex metering circuitry; and they have a remarkably long shelf life—up to 10 years, and even longer under refrigeration. There’s only one problem with mercury batteries, but it’s a killer—they’re extremely toxic, especially when they’re incinerated with other waste, or wind up in landfills. That’s why the EPA sponsored Mercury-Containing and Rechargeable Battery Management Act of 1996 prohibited the use of mercury in all types of consumer batteries, and mercury-free batteries such as alkaline and silver-oxide cells became the de facto international standard for most camera batteries.

Banned Battery: Varta V625PX mercury cell, like others of its ilk, is no longer made or sold worldwide, but there are excellent alternatives.
The Great Battery Conversion Kerfuffle
The basic problem for photographers using one of the scores of vintage cameras and meters designed to take 1.35v mercury cells is that the currently available alkaline and silver oxide cells that will fit their devices’ battery compartments put out a higher voltage—generally in the 1.5 -1.6v range. Installing a higher voltage button battery directly in place of the defunct mercury cell won’t usually damage the device, but the meter readings can be way off—as much as 2-3 stops. However, if you check the reading against a camera or meter of known accuracy and it’s consistently, say, one stop off on the overexposure side, you can compensate by setting a one stop lower ISO—200 instead of 400. But before you simply set it and forget, check the reading at several different light levels to make sure its response is linear.
Bottom line: The best way to ensure long-term metering accuracy with today’s batteries in your vintage camera or meter is to make sure it puts out the same voltage the device was designed for. The most practical solutions: 1. Use current zinc-air cells of the correct size and voltage. 2. Have a competent repairman wire a voltage-modulating diode into your camera’s metering circuitry. 3. Purchase an MR-9 Mercury Battery Adapter that has built-in voltage-reducing circuitry. All three methods work well, but each has its upsides and downsides.

MRB625 1.35v zinc-air cell replaces PX635, PX13, and MR-9 mercury cells
Zinc-Air Batteries
The great advantages of Zinc-Air batteries are that they provide a stable voltage, have a storage life of up to 10 years when not activated, and are literally drop-in replacements for their respective mercury cells—no adapters required. The downside is that they must be activated by removing a pull tab to expose holes in the casing that allow ambient air to interact with the electrolyte to generate a current. If these holes remain open when the camera or meter is stored, the life of the cells can be substantially reduced—from months to weeks or even days depending on the precise storage conditions. Removing the zinc-air battery or batteries and replacing the tab(s) when they’re not in use can definitely prolong their life, but it’s an off-putting inconvenience. User reviews on zinc-air cells run the gamut from extremely positive to harshly negative, so as they say, “Your experience may vary.”

MRB625 Wein Cell, its most popular zinc-air cell, in its original packing
Wein Cells
By far the most popular brand of zinc air cell is the Wein Cell that’s available at numerous online selling sites and major photo specialty retailers. The best-selling version is the Wein MRB625 1.35v Zinc-Air Battery (about $5.00) that’s equivalent to the discontinued PX625, PX13, PX67, and MR-9 mercury cell used in many vintage devices. Others include the 1.35v Wein MRB675 that replaces the PX675, and the 1.35v Wein MRB400 that replaces RM400R and V400PX cells. For more tech info, contact Micro-Tools Division of Fargo Enterprises in Vacaville, CA. Phone: 707-446-1120.
Modifying the camera to work with available batteries
Wiring a voltage-modulating diode into your camera’s metering circuitry so it gives correct exposure readings with currently available batteries has the great advantage of being a permanent fix that requires no modification of the battery compartment, spacers, etc. for the new batteries to fit. The downsides: most consumers will have to send the camera (or meter) to a competent repair shop to do the job, and it typically costs $50-$60 plus the cost of 2-way shipping.
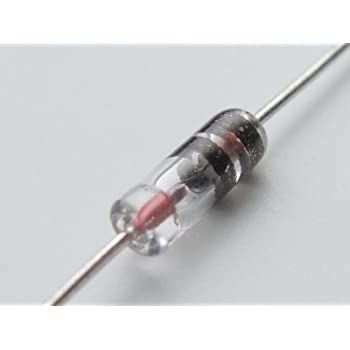
This 1N34 diode is permanently wired into camera's metering circuitry to allow the use of available silver-oxide or alkaline cells going forward.
The most common diodes used in this operation are the 1N34 germanium diode, which drops the battery voltage by 0.3 to 0.35v, the Schottky diode that drops the voltage by about 0.25v, and the silicon diode 1N4002, which drops voltage by 0.7v and is suitable for applications requiring two 1.5v batteries. All these diodes are wired in series with the battery, and they’ll work with silver-oxide cells (preferable because they deliver a steadier voltage) or alkaline cells (which drop voltage at a faster rate and should be replaced at shorter intervals—every year at least). Due to variations in metering circuitry, most competent repairman make sure to calibrate the metering system after modification to ensure that the readings are still within spec —make sure yours does so before sending your camera in.
The MR-9 Battery Adapter
Made in Japan by Kanto, the MR-9 Voltage Reducing Converter has an imbedded microelectronic adjusting circuit that drops the 1.55v output of a 386 silver-oxide cell (or equivalent) to the 1.35 volts required by cameras or meters designed for mercury cells. Just insert a 386 cell into the adapter, and the entire unit fits directly into virtually any battery compartment designed for PX625, PX13, EPX625, MR9 or equivalent batteries. The MR-9 adapter will also work with the slightly deeper, higher capacity SR44 or 357 silver-oxide cells, providing the battery compartment has spring contacts that will allow the battery- compartment cover to be screwed down fully. The upsides: It’s a do it yourself solution, and the adapter itself a one-time purchase that has no moving parts and can be used for as long as it’s needed.
%20and%20adapter%20.jpg/:/rs=w:1440,h:1440)
MR-9 Battery Adapter alone (left), and with 386 silver-oxide cell in place (right) prior to inserting it in camera's battery compartment.
The MR-9 Mercury Battery Adapter ($39.95) is distributed in the U.S. by C.R.I. S., 190 North 54th St, Chandler AZ 85226, Tel # 800-799-0293, email: CRISCAM.COM. The MR-9 is the only battery adapter C.R.I.S. currently has in stock, but they also list the V27PX adapter for four 386 cell applications, the HM-4N Adapter that accommodates one 4SR44 battery, and the H-B Adapter for the 377 battery They’ll all be available when stocks are replenished.
Warning: Not all adapters are created equal
There are many so-called mercury battery adapters offered on major selling sites such as Amazon that only adapt smaller batteries to fit larger battery compartment receptacles and do nothing whatsoever to alter the voltage of the inserted batteries. They may be of some value in other applications, but steer clear them if you’re aiming to use current silver-oxide or alkaline batteries in a device that was designed for mercury batteries. If you’re tempted, check out the scathing reviews on Amazon before you click “buy.”

Pretty but (mostly) useless, cheap size adapters like these let you put smaller batteries in bigger battery compartments but they don't alter the voltage.
More than you wanted to know about mercury battery solutions
For a fascinating 14-page screed that covers this topic in excruciating detail and includes a number of ingenious labor-intensive DIY solutions, copy and paste the following link to the PDF into your browser, and if you have enough paper, ink, and motivation, print it out.
http://www.buhla.de/Foto/batt-adapt-US.pdf
A handy guide to non-toxic workarounds for vintage cameras & meters
By Jason Schneider
Beginning in the early 1960s, battery powered CdS, and later, SBC cells, were steadily replacing bulkier, less sensitive selenium cells in the metering systems built into cameras and handheld exposure meters. Compared to their selenium cell-based counterparts, these new battery powered meters and metering systems worked at far lower light levels, had narrower acceptance angles, making it easier to get accurate readings from shooting position, didn’t require a delicate micro-ammeter, and could be integrated more compactly into camera bodies without the telltale honeycomb grid. However, unlike selenium cells that generate a measurable current on their own when exposed to light these new meters required batteries.
Initially, most camera and meter manufacturers turned to mercury batteries, technically known as mercuric-oxide cells, to power these new meters and metering systems because they offer a host of operational advantages. Mercury batteries deliver a very constant voltage over their long lifespan and then die suddenly, virtually eliminating the potential for inaccurate readings; they don’t require complex metering circuitry; and they have a remarkably long shelf life—up to 10 years, and even longer under refrigeration. There’s only one problem with mercury batteries, but it’s a killer—they’re extremely toxic, especially when they’re incinerated with other waste, or wind up in landfills. That’s why the EPA sponsored Mercury-Containing and Rechargeable Battery Management Act of 1996 prohibited the use of mercury in all types of consumer batteries, and mercury-free batteries such as alkaline and silver-oxide cells became the de facto international standard for most camera batteries.

Banned Battery: Varta V625PX mercury cell, like others of its ilk, is no longer made or sold worldwide, but there are excellent alternatives.
The Great Battery Conversion Kerfuffle
The basic problem for photographers using one of the scores of vintage cameras and meters designed to take 1.35v mercury cells is that the currently available alkaline and silver oxide cells that will fit their devices’ battery compartments put out a higher voltage—generally in the 1.5 -1.6v range. Installing a higher voltage button battery directly in place of the defunct mercury cell won’t usually damage the device, but the meter readings can be way off—as much as 2-3 stops. However, if you check the reading against a camera or meter of known accuracy and it’s consistently, say, one stop off on the overexposure side, you can compensate by setting a one stop lower ISO—200 instead of 400. But before you simply set it and forget, check the reading at several different light levels to make sure its response is linear.
Bottom line: The best way to ensure long-term metering accuracy with today’s batteries in your vintage camera or meter is to make sure it puts out the same voltage the device was designed for. The most practical solutions: 1. Use current zinc-air cells of the correct size and voltage. 2. Have a competent repairman wire a voltage-modulating diode into your camera’s metering circuitry. 3. Purchase an MR-9 Mercury Battery Adapter that has built-in voltage-reducing circuitry. All three methods work well, but each has its upsides and downsides.

MRB625 1.35v zinc-air cell replaces PX635, PX13, and MR-9 mercury cells
Zinc-Air Batteries
The great advantages of Zinc-Air batteries are that they provide a stable voltage, have a storage life of up to 10 years when not activated, and are literally drop-in replacements for their respective mercury cells—no adapters required. The downside is that they must be activated by removing a pull tab to expose holes in the casing that allow ambient air to interact with the electrolyte to generate a current. If these holes remain open when the camera or meter is stored, the life of the cells can be substantially reduced—from months to weeks or even days depending on the precise storage conditions. Removing the zinc-air battery or batteries and replacing the tab(s) when they’re not in use can definitely prolong their life, but it’s an off-putting inconvenience. User reviews on zinc-air cells run the gamut from extremely positive to harshly negative, so as they say, “Your experience may vary.”

MRB625 Wein Cell, its most popular zinc-air cell, in its original packing
Wein Cells
By far the most popular brand of zinc air cell is the Wein Cell that’s available at numerous online selling sites and major photo specialty retailers. The best-selling version is the Wein MRB625 1.35v Zinc-Air Battery (about $5.00) that’s equivalent to the discontinued PX625, PX13, PX67, and MR-9 mercury cell used in many vintage devices. Others include the 1.35v Wein MRB675 that replaces the PX675, and the 1.35v Wein MRB400 that replaces RM400R and V400PX cells. For more tech info, contact Micro-Tools Division of Fargo Enterprises in Vacaville, CA. Phone: 707-446-1120.
Modifying the camera to work with available batteries
Wiring a voltage-modulating diode into your camera’s metering circuitry so it gives correct exposure readings with currently available batteries has the great advantage of being a permanent fix that requires no modification of the battery compartment, spacers, etc. for the new batteries to fit. The downsides: most consumers will have to send the camera (or meter) to a competent repair shop to do the job, and it typically costs $50-$60 plus the cost of 2-way shipping.

This 1N34 diode is permanently wired into camera's metering circuitry to allow the use of available silver-oxide or alkaline cells going forward.
The most common diodes used in this operation are the 1N34 germanium diode, which drops the battery voltage by 0.3 to 0.35v, the Schottky diode that drops the voltage by about 0.25v, and the silicon diode 1N4002, which drops voltage by 0.7v and is suitable for applications requiring two 1.5v batteries. All these diodes are wired in series with the battery, and they’ll work with silver-oxide cells (preferable because they deliver a steadier voltage) or alkaline cells (which drop voltage at a faster rate and should be replaced at shorter intervals—every year at least). Due to variations in metering circuitry, most competent repairman make sure to calibrate the metering system after modification to ensure that the readings are still within spec —make sure yours does so before sending your camera in.
The MR-9 Battery Adapter
Made in Japan by Kanto, the MR-9 Voltage Reducing Converter has an imbedded microelectronic adjusting circuit that drops the 1.55v output of a 386 silver-oxide cell (or equivalent) to the 1.35 volts required by cameras or meters designed for mercury cells. Just insert a 386 cell into the adapter, and the entire unit fits directly into virtually any battery compartment designed for PX625, PX13, EPX625, MR9 or equivalent batteries. The MR-9 adapter will also work with the slightly deeper, higher capacity SR44 or 357 silver-oxide cells, providing the battery compartment has spring contacts that will allow the battery- compartment cover to be screwed down fully. The upsides: It’s a do it yourself solution, and the adapter itself a one-time purchase that has no moving parts and can be used for as long as it’s needed.
%20and%20adapter%20.jpg/:/rs=w:1440,h:1440)
MR-9 Battery Adapter alone (left), and with 386 silver-oxide cell in place (right) prior to inserting it in camera's battery compartment.
The MR-9 Mercury Battery Adapter ($39.95) is distributed in the U.S. by C.R.I. S., 190 North 54th St, Chandler AZ 85226, Tel # 800-799-0293, email: CRISCAM.COM. The MR-9 is the only battery adapter C.R.I.S. currently has in stock, but they also list the V27PX adapter for four 386 cell applications, the HM-4N Adapter that accommodates one 4SR44 battery, and the H-B Adapter for the 377 battery They’ll all be available when stocks are replenished.
Warning: Not all adapters are created equal
There are many so-called mercury battery adapters offered on major selling sites such as Amazon that only adapt smaller batteries to fit larger battery compartment receptacles and do nothing whatsoever to alter the voltage of the inserted batteries. They may be of some value in other applications, but steer clear them if you’re aiming to use current silver-oxide or alkaline batteries in a device that was designed for mercury batteries. If you’re tempted, check out the scathing reviews on Amazon before you click “buy.”

Pretty but (mostly) useless, cheap size adapters like these let you put smaller batteries in bigger battery compartments but they don't alter the voltage.
More than you wanted to know about mercury battery solutions
For a fascinating 14-page screed that covers this topic in excruciating detail and includes a number of ingenious labor-intensive DIY solutions, copy and paste the following link to the PDF into your browser, and if you have enough paper, ink, and motivation, print it out.
http://www.buhla.de/Foto/batt-adapt-US.pdf
